Ethylene Oxide Sterilization Of Medical Devices A Review
8 0 0 0 0. Only residual ethylene oxide remains on nasal swabs after sterilization.

Pdf Residual Ethylene Oxide In Medical Devices Effects And Estimation Methods An Overview
Equipment and Supplies Ethylene Oxidetoxicity.

Ethylene oxide sterilization of medical devices a review. By Gisela C C Mendes Teresa R S Brandão Cristina L M Silva. Read more related scholarly scientific articles and abstracts. Mendes MD Teresa R.
This paper describes the progress in terms of EO sterilization and concludes that it remains a promising field to explore and develop. Ethylene oxide EO is a well-known sterilizing agent. American journal of infection control.
This paper describes the progress in. Sterilization of medical devices packaging The effect on polymer properties color A wide variety of sterilization methods are used in the medical industry including electron beam e-beam irradiation gamma irradiation ethylene oxide EtO autoclave and low. The series of standards governing the biologic testing of MDs include the International Organization for Standardization ISO 10993-7 Biological Evaluation of MDsEthylene Oxide Sterilization Residuals which specifies the allowable limits of EO and ethylene chlorohydrin by categorization of products based on examination of toxicologic risk of the residue to the patient according to the length of the time the patient is likely to be exposed to the device.
Journal of Biomaterials Applications 1988 3. Sterilization of medical devices. However only recently has its use significantly emerged based on its range of applications in the field of new medical device development and sterilization.
Sterilization of medical devices. If the review finds unacceptable risk levels from commercial sterilizers EPA should revise the National Emissions Standards for Hazardous Air Pollutants for sources that emit ethylene oxide. This timely review provides a succinct overview of VH2O2 in gaseous sterilization and addresses its applicability for terminal sterilization of medical devices.
Brandao PhD and Cristina L. Authors D J Dempsey 1 R R Thirucote. Ethylene Oxide Sterilization for Medical Devices.
A review J Biomater Appl. 2654354 Indexed for MEDLINE Publication Types. Ethylene oxide sterilization of medical devices.
However only recently has its use significantly emerged based on its range of applications in the field of new medical device development and sterilization. Advanced course assumes that participants are experienced in working with an established ethylene oxide sterilization process but are now challenged. Affiliation 1 Thermedics.
A Review Donald J. Review of the Controversy Surrounding the Use of Ethylene Oxide in Medical Device Sterilization substances that could lead to developmental complications in juveniles. Review Ethylene Oxide Gas Sterilization of Medical Devices HIDEHARU SHINTANI Department of Science and Engineering Chuo University 1-13-27 Kasuga Bunkyo Tokyo 112-0003 Japan Received 25 March 2015Accepted 2 February 2016 Ethylene oxide gas is an agent in the sterilization of medical devices due to its effectiveness.
Medigy surfaces the worlds best crowdsourced health tech offerings with social interactions and peer reviews. Dempsey DJ1 Thirucote RR. However only recently has its use significantly emerged based on its range of applications in the field of new medical device development and sterilization.
For many medical devices sterilization with ethylene oxide may be the only method that effectively sterilizes and does not damage the device during the sterilization process. The Environmental Protection Agencys Office of Inspector General is advising EPA to conduct a fresh review of residual cancer risks from ethylene oxide emissions associated with medical device sterilization facilities. Ethylene oxide sterilization of medical devices.
Currently major hospitals as well as medical device manufacturers still use EO sterilization 282930 31. A review Gisela C. Sterilization of Medical Devices.
Ethylene oxide is used for sterilizing medical equipment including nasal swabs but its removed before use - Health Feedback. In addition to this they modified their 1987 determination that EO leads to reproductive issues in. Ethylene oxide sterilization of medical devices.
Ethylene oxide sterilization is regulated so that medical devices sterilised using ethylene oxide are safe to use. Ethylene oxide EO is a well-known sterilizing agent. Ethylene oxide EO has been widely used to sterilize medical devices.
Sterilisation the sterilisation of biomaterials and medical devices using steam and dry heat ionising radiation and ethylene oxide is reviewed. Silva PhD Porto Portugal Ethylene oxide EO is a well-known sterilizing agent. 1Thermedics Inc Woburn MA 01888.
A range of non-traditional sterilisation techniques such as hydrogen peroxide gas plasma ozone and steam formaldehyde is then discussed together with research in sterilisation and. It also describes underappreciated factors such as the occurrence of nonlinear microbial inactivation kinetic plots that may dictate a need to develop a new standard approach to validate VH2O2 for terminal sterilization of medical devices. Sterilization of medical devices.
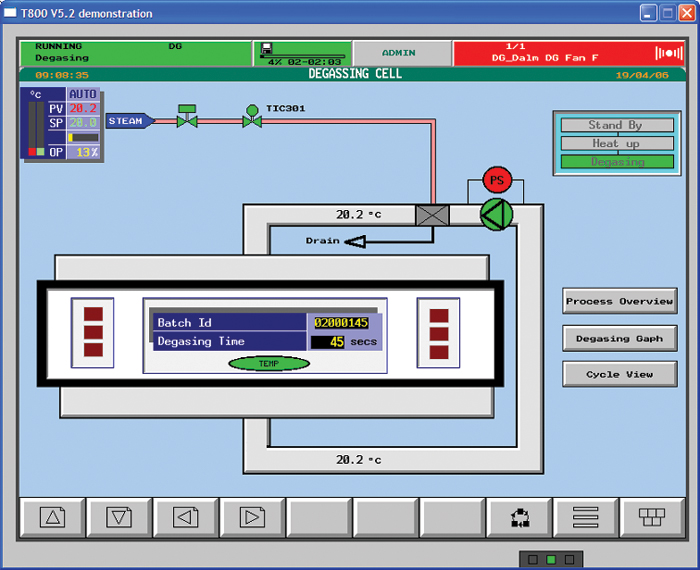
Ethylene Oxide Eto Sterilization Process Eurotherm By Schneider Electric

Ethylene Oxide Sterilization Advamed

Pdf Residual Ethylene Oxide In Medical Devices And Device Material

Pdf Kinetics Of Ethylene Oxide Desorption From Sterilized Materials

Pdf Ethylene Oxide Potential Toxicity

Pdf Ethylene Oxide Sterilization Of Medical Devices A Review Teresa Brandao Academia Edu
Ethylene Oxide Sterilization Validation Pacific Bio Labs Inc

Pdf Ethylene Oxide Gas Sterilization Of Medical Devices Semantic Scholar

Ethylene Oxide Sterilization Validation Pacific Bio Labs Inc
Eq Sterilization Shenzhen King Medical Packaging Sterilization Service Co Ltd

What Is Ethylene Oxide Uses Safety Technology Video Lesson Transcript Study Com
Seeking Sterilization Solutions A Look At Ethylene Oxide Medical Product Outsourcing
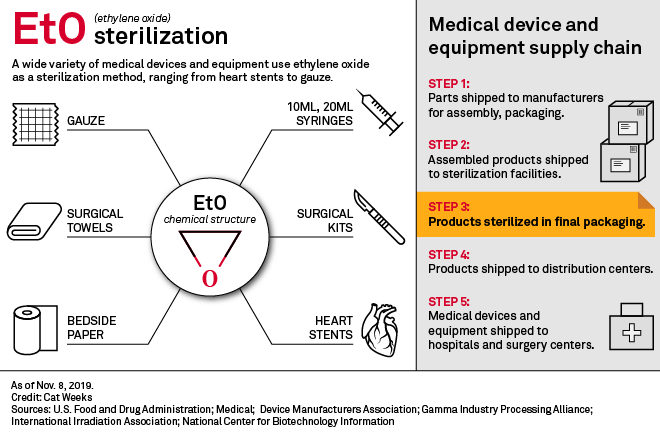
Amid Threat Of Device Shortages Us Fda Works To Limit Ethylene Oxide Use S P Global Market Intelligence

Pdf Residual Ethylene Oxide In Medical Devices Effects And Estimation Methods An Overview
Eq Sterilization Shenzhen King Medical Packaging Sterilization Service Co Ltd
![]()
U S Fda Statement On New Steps To Advance Innovation In Medical Device Sterilization With Ethylene Oxide Fda Reporter

How To Choose The Best Medical Device Sterilization Method Xtalks

0 Response to "Ethylene Oxide Sterilization Of Medical Devices A Review"
Posting Komentar