Ethylene Oxide Production Process
Annual worldwideproductioncapacityisca15 106 t. The EO production process is energy intensive and creates direct CO 2 emissions making it critical to deploy transformative technologies that go beyond business as usual.
Https Onlinelibrary Wiley Com Doi Pdf 10 1002 Cben 201600025
Selection of X values that satisfy the target values of multiple Y variables are searched and simulations for the selected X values are then repeated.

Ethylene oxide production process. METEOR EO-RETRO 2000 a more efficient catalyst Dow developed the Most Effective Technology for Ethylene Oxide Reaction METEOR catalysts to increase the performance and. PROCESS DESCRIPTION WITH BLOCK FLOW DIAGRAM AND DESCRIPTION OF QUALIFIED PROPERTY ETHYLENE OXIDE PROCESS DESCRIPTION Ethylene oxide EO C 2H 4O is a colorless flammable gas or liquid. So one of the components that we need to analyze to then determine and control the efficiency of the scrubber is to measure how much ethylene again is being scrubbed or removed from the reactor effluent.
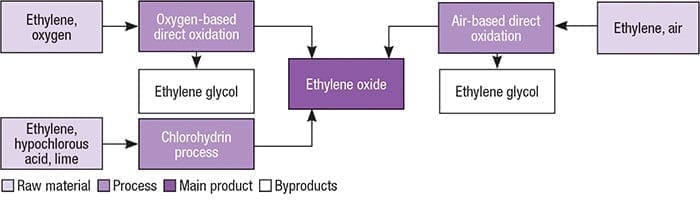
Ethylene compressed oxygen and recycle gas are mixed and fed to a multi-tubular catalytic reactor. Ethylene oxide is produced by the catalytic oxidation of ethylene over a silver-containing catalyst. 121 Production Production of ethylene oxide began in 1914 by the chlorohydrin process the main method used until 1937 in which ethylene chlorohydrin is converted to ethylene oxide by reaction with calcium oxide.
Ethylene oxide production is an old process that has gone through one major change throughout its life. The production of ethylene chlorohydrin resulted in the. Ethylene oxide EO was initially manufactured using ethylene chlorohydrin as an intermediate but this route has been superseded by the direct oxidation of ethylene with air or oxygen.
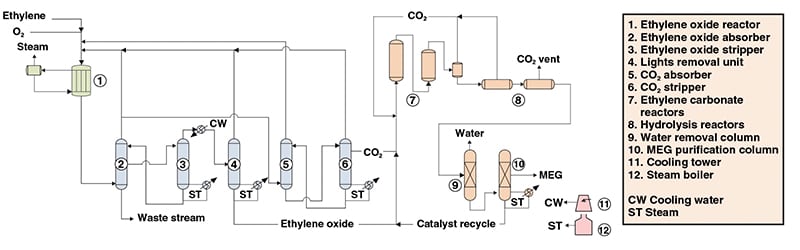
CO 2 scrubber and CO 2 de-scrubber. The typical yield of this reaction is up to 80 90. All the modern industrial EO production processes are based on the gas-phase oxidation of ethylene in tubular reactors carried out at 220300C and 13 MPa with the use of Al 2 O 3.
The main reactor consists of thousands of catalyst tubes in bundles. Currently ethylene oxide is produced by direct oxidation of ethylene with air or oxygen. Now nearly all the worlds EO capacity is based on direct oxidation with oxygen generally preferred.
In 1931 LEFORT 9 discovered the direct catalytic oxidation of ethylene 74-85-1 which gradually superseded the chlorohydrin process. However now it is made by the direct oxidation of ethylene with air in the presence of a catalyst of silver supported on α-alumina Eq. Together with ethylene oxide CO2 H2O and heat is generated as well.
The production of ethylene oxide on a commercial scale is attained with the unification of the following unit processes. Yes because ethylene is also produced is part of the reactor effluent because we know we dont produce just ethylene oxide or other gases. Production of ethylene oxide is the next largest single use of ethylene.
Steps of oxygen-based ethylene oxide processing Feed of ethylene and oxygen into the reactor and oxidation of ethyl-ene by passing through the reactor bundles of tubes packed with the catalyst material at 200 to 300 C. The invention relates to a process for the production of ethylene oxide comprising the steps of producing ethylene resulting in a stream comprising ethylene and ethane. It used to be produced via the chlorohydrin process as propylene oxide still is.
Ethylene Oxide EO Production and Manufacturing Process. It is an extremely versatile chemical intermediate. Therefore the X will be selected by a small number of simulations.
Initially this was accomplished by the World War I. The basic goal is still the same to react ethylene and oxygen either directly or indirectly to produce ethylene oxide and several undesirable by-products. Stripping and distillation column.
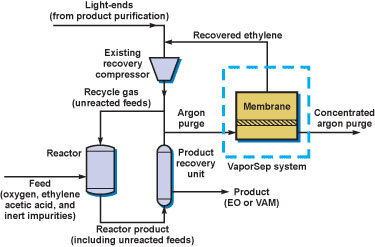
In a process for the direct oxidation of ethylene to ethylene oxide ethylene is recovered from normally vented gas by contacting first with an activated carbon adsorbent then by pressure swing. Production and importance of ethylene oxide have steadily grown. Ethylene oxide can be produced by reacting oxygen O 2 and ethylene C 2 H 4 at temperatures of 200 300C and pressures of 10 20 bara.
We verify the effectiveness of this method by simulating an ethylene oxide production plant. A side reaction oxidizes ethylene to carbon dioxide and water.

Improved Process Design And Optimization Of 200 Kt A Ethylene Glycol Production Using Coal Based Syngas Sciencedirect
Https Onlinelibrary Wiley Com Doi Pdf 10 1002 Cben 201600025
Https Onlinelibrary Wiley Com Doi Pdf 10 1002 Cben 201600025
New Catalytic Process For Production Of Olefins Oil Gas Portal

Process Flow Diagram Of The Cebc Process Table 3 Lists The Simulation Download Scientific Diagram
Https Onlinelibrary Wiley Com Doi Pdf 10 1002 Cben 201600025

Chloride Mediated Selective Electrosynthesis Of Ethylene And Propylene Oxides At High Current Density Science

Improved Process Design And Optimization Of 200 Kt A Ethylene Glycol Production Using Coal Based Syngas Sciencedirect

Process Flow Diagram Of The Cebc Process Table 3 Lists The Simulation Download Scientific Diagram
Technology Profile Ethylene Oxide Production From Ethylene Chemical Engineering Page 1
Https Www Ugr Es Tep028 Pqi Descargas Industria 20quimica 20organica Tema 5 Oxido Etileno A10 117 Pdf
New Catalytic Process For Production Of Olefins Oil Gas Portal

Dynamic Optimal Design Of An Industrial Ethylene Oxide Eo Reactor Via Differential Evolution Algorithm Sciencedirect
Ethylene Glycol Production Chemical Engineering

Improved Process Design And Optimization Of 200 Kt A Ethylene Glycol Production Using Coal Based Syngas Sciencedirect

Ethylene An Overview Sciencedirect Topics
Cpmai Chemicals Petrochemicals Manufacturer S Association Of India

0 Response to "Ethylene Oxide Production Process"
Posting Komentar